Breast augmentation is still one of the most popular plastic surgery procedures in the United States as more than 300,000 patients in the United States had breast augmentation performed last year. One of the most popular methods of breast augmentation is having breast implants placed to enhance the appearance and size of the breasts. However, many women are making the decision to have their breast implants removed from their body. Some women decide that the implants are no longer the size breasts they want to have while others report being sickened by a so-called “breast implant illness” which includes symptoms such as headaches, fever, rashes, chronic fatigue and joint pain. The reason the illness is “so-called” is because of the fact that the illness is not officially recognized by the FDA or doctors. In addition, female patients with textured breast implants run the risk of suffering from a type of lymphoma called breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).
Some of the most common reasons for breast implant removal include:
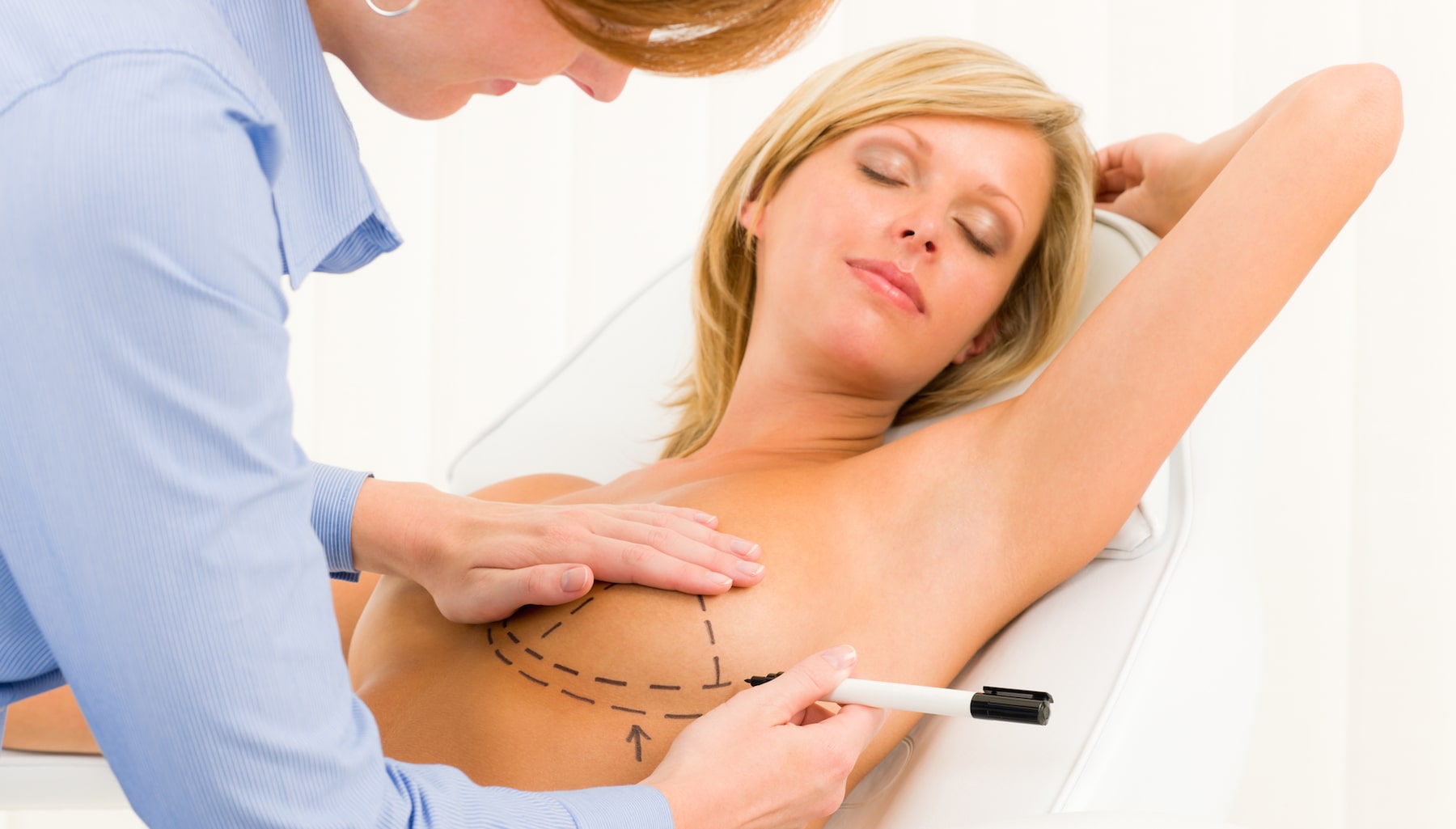
Breast implant removal can address multiple concerns such as a desire to change the breast size, implant malfunction and capsular contracture (a capsule of scar tissue that is hard and contracts around the implant). The breast implant removal process usually involves making an incision through the original incision where the breast implants were placed. The new incision is made in order to remove the breast implant. It should be noted that patients that had the implants placed via an underarm incision (axillary incision) normally need a new incision created under the breast. Besides removing the breast implants, the surgeon will also remove any scar tissue that is present around the area where the implants were placed. Sutures, clips or tape are used to close the new incision.
As mentioned above, patients with textured breast implants are at risk for BIA-ALCL. While not every person with textured breast implants will develop this form of lymphoma, it is important for patients to watch for signs of fluid collection or lumps around the breast implants. If these issues arise, the person should schedule a consultation appointment with a doctor that is experienced in identifying and treating BIA-ALCL. It is currently recommended that patients with textured silicone breast implants get a breast MRI every two to three years so medical professionals can monitor for implant ruptures and any changes that occur around the breast implants.
The breast implant removal recovery process is generally smoother than the initial breast implant surgery. Most women can return to work after about five days and they normally experience minimal discomfort after the surgery. There will be some post-op swelling, soreness and bruising.
Patients that have the breast implants removed due to capsular contracture will experience more discomfort and a longer recovery time. Any scars from the surgery will fade as time passes but they will not disappear completely. Patients should avoid any heavy lifting or strenuous exercise for several weeks after the surgery. In order to enjoy the best recovery period, patients should follow any instruction provided to them by their surgeon.
When it comes to breast implant illness, the FDA says there is no scientific evident that supports the existence of breast implant illness. They did issue a warning earlier this year that some patients do experience “systemic symptoms” that might go away “when their breasts implants are removed”.
In addition, the FDA held a hearing on the topic of breast implant safety back in March. The hearing was attended by representatives from all four American breast implant manufacturers and they include Sientra, Mentor Worldwide, Allergan and Ideal Implant. Their statements at the hearing actually seemed to argue against their own customers since all four companies claimed that breast implant illness does not exist.
The FDA is also ordering breast implants to have better warning labels because, as of January, the government agency has received around 370 reports of illnesses related to breast implants. Due to the large number of reports related to breast implant illness, the FDA is recommending a more in-depth investigation into the concerns of patients caused by breast implant illness.
The issue of breast implant illness, and whether or not it is actually an issue caused by breast implants placed in the body of patients, is not likely to be solved or given a definitive answer anytime in the near future. Until the FDA makes a definitive ruling on breast implant illness, patients are advised to consult with a board-certified doctor if they have any issues or concerns related to their breast implants.
MA